Background: Early progression within 24 months (POD24) of initial immunochemotherapy was repeatedly confirmed to be associated with poor survival in follicular, diffuse large B-cell and mantle-cell lymphomas, identifying its high-risk subgroup with different disease biology (Casulo C, JCO 2015; Maurer JM, JCO 2015; Bond DA, Blood Adv 2021). Little is known about the POD24 incidence and clinical impact in Hodgkin lymphoma (HL) patients, and current prognostic schemes have been designed with longer survival endpoints (Rodday AM, JCO 2022).
Methods: This single-center study comprised patients (pts) with classic HL (cHL) treated with frontline chemotherapy prospectively enrolled in the Czech Hodgkin Lymphoma Study Group database in 1993-2020 (database lock was done on 15. June 2023). Early event was defined as progression, relapse, or death related to progressive HL within 24 months after the date of diagnosis. Overall (OS) and progression-free survival (PFS) were calculated from the date of diagnosis. To evaluate the association between early POD and OS from a risk-defining event, that is, survival from the time of POD for early progressors (POD24 group) or from 2 years after diagnosis for the reference group (noPOD24 group), was calculated. In total, we have identified 417 pts with cHL diagnosed from 1993 to the end of 2020. Ten pts have been excluded from the analysis because of early death (<24 months) with no recorded disease progression.
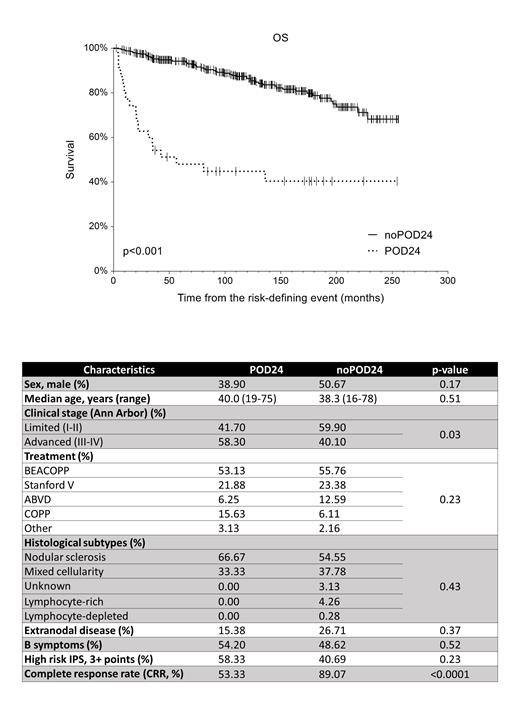
Results: The analyzed cohort consisted of 407 pts: 205 females and 202 males with a median age of 35 years (min 16, max 78) with limited disease (stage I-II) in 58% and advanced disease (stage III-IV) in 42%. Histological subtypes were as follows: nodular sclerosis in 53.1%, mixed cellularity in 35.6%, lymphocyte-rich in 3.7%, and lymphocyte-depleted in 0.3%. Subtype was not available in 7.3% of the pts. Extranodal disease and B symptoms were present in 25% and 49%, respectively. 42% of patients had high-risk IPS of 3 or more points. Patients received BEACOPP in 55.5%, Stanford V in 23%, ABVD in 12%, and COPP in 7%. Other treatments were used in 2,5% of the cases. The complete and partial remission rates after frontline treatment were 88.7% and 8.6%, respectively, and only 3% of the pts did not respond to therapy.
After a median follow-up of 12.1 years, a cumulative incidence of progression/relapse is 0.14 (95%CI 0,10-0.18). The POD24-event occurred in 36 pts (8.8%) and 38 (9.3%) patients relapsed after 2 years. The entire group's 5-y PFS and 5-y OS reached 81.0% (95% CI 0.77-0.85) and 91.4% (95% CI 88.6-0.94), respectively.
The 5-y OS since the risk-defining event was 48.1% (95% CI 31.4-64.8) and 94.2% (95% CI 0.92-96.7) in the POD24 vs noPOD24 group (p<0.001, HR=5.35), Fig 1. When comparing 12 baseline clinical and laboratory characteristics between POD24 (n=35) with noPOD24 controls (n=371), we have found no major differences (Tab 1). There was a trend in more frequent POD24 in the patients with massive mediastinal tumor (p=0.07) and those with advanced clinical stage (p=0.047). The spectrum of the induction regimes was distributed equally (p=0.26).
Conclusions: Early progression of the disease is rare; however, a catastrophic event in HL resulting in more than five times higher risk of death. Baseline disease characteristics - besides the clinical stage - do not predict the risk of later POD24 events. Large independent validation may enhance the validity of these data and help create a prognostic tool for POD24 prediction. This event should be considered an important endpoint in Hodgkin lymphoma clinical trials in the future.
Acknowledgements: Supported by MH CZ - DRO (FNOL, 00098892), IGA_LF_2023_005 and AZV NU22-03-00182.
Disclosures
Bachanova:Allogene: Membership on an entity's Board of Directors or advisory committees; Gamida Cell: Research Funding; Incyte: Research Funding; ADC: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Citius: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Other: DSMB.